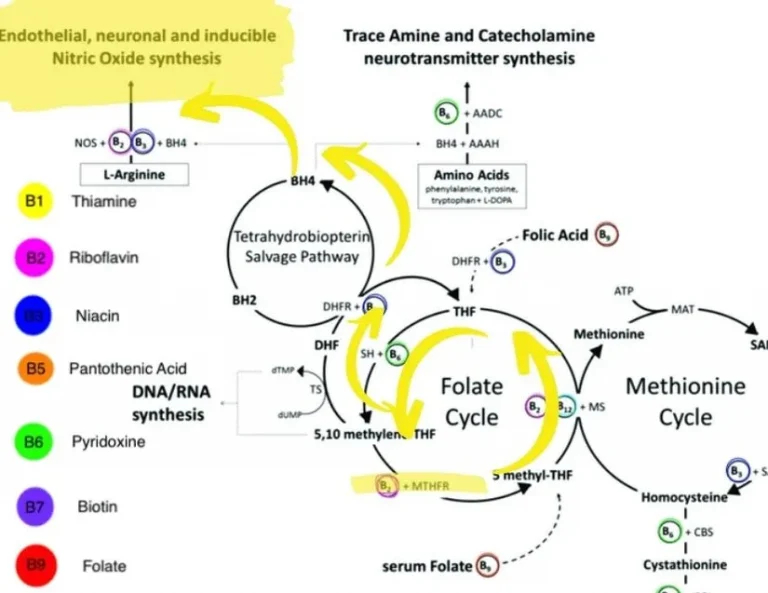
MTHFR is well known for causing heart health issues. It’s directly responsible for raising homocysteine levels, it’s implicated in troublesome blood clotting, and it’s indirectly responsible for making it harder to make adequate nitric oxide. All of this is compounded…